HISTORY OF THE PERIODIC TABLE OF ELEMENTS
The periodic table of elements is familiar to anyone that ever entered a laboratory or classroom. It has no rival in its ability to systematize and rationalize known chemical facts, predict new elements, or suggest fertile areas for further studies. This ingenious and highly functional arrangement of chemical elements was developed by several European scientists in the 19th century. What follows is a brief history of the creation of the periodic table of elements.
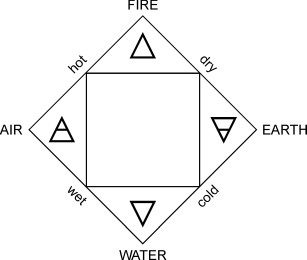
The old Greek philosophers Tales (624-546), Anaximander (610-546), and Heraclites (540-480) claimed that all matter was made up of one fundamental principle - or element. Tales believed that this element was water, his student Anaximander thought it was air, and Heraclites speculated it was fire. Empedocles (490-430) believed that all matter was made up of four elements: air, earth, fire, and water, and that by mixing those elements in various ratios one could create every substance found in nature. A hundred years later Aristotle (384-322) embraced his idea and added that, each of the four elements has four basic properties: hot, cold, dry, and wet. This appeared to be quite reasonable since burnt wood decomposed into three elements: water, air, and earth. The only thing kept from the Greek idea of element was that each element has characteristic properties.
It took almost 2000 years for someone to seriously attack Aristotle's theory of the four elements and the somewhat more contemporary three alchemical principles (mercury, sulphur, and salt). This was done in 1661 by the "father of modern chemistry ", English-Irish chemist and naturalist Robert Boyle (1627-1691), in his book "The Sceptical Chymist". Without going into the nature and number of elements, Boyle defined each element as a simple substance, a building block from which more complex things are made. Complex bodies are made from elements and can be reduced to those elements through chemical analysis.
The first table of simple chemical substances was presented in 1789 by the french chemist Antoine-Laurent Lavoisier (1743-1794) in his book "Traité Élémentaire de Chimie ". In this classic work, Lavoisier made sure to give concise explanations of both his own work, and the work of his predecessors. He clarified the distinction between elements and compounds, and also assisted in developing the modern system of chemical terminology. His table of elements, simple substances that cannot be further split and from which all other matter was formed, contained 33 elements divided into four groups: gasses, non-metals, metals, and soil (Table 1). Ironically, given his enormous contribution to the overthrow of the phlogiston theory, he listed light and heat among the elements. Lavoisier's name is inextricably linked with the very foundations on which modern science lies: it is considered that he did for chemistry what Isaac Newton (1642.-1727.) did for physics.
| Substances simples qui appartiennent aux trois règnes et qu'on peut regarder comme les éléments des corps | Substances simples non-métalliques oxydables et acidifiables | Substances simples métalliques oxydables et acidifiables | Substances simples salifiables terreuses | |
|---|---|---|---|---|
|
Lumière Calorique Oxygène Azote Hydrogène |
Soufre Phosphore Carbone Radical muriatique Radical fluorique Radical boracique |
Antimoine Argent Arsenic Bismuth Cobalt Cuivre Étain Fer Manganèse |
Mercure Molybdène Nickel Or Platine Plomb Tungstène Zinc |
Chaux (CaO) Magnésie (MgO) Barite (BaO) Alumine (KAl(SO4)2·12H2O) Silice (SiO2) |
The English chemist John Dalton (1766-1844) proposed his principles of atomic theory in 1803, suggesting that all elements are composed of tiny, indestructible particles called atoms, all of which are equal and weigh exactly the same. Atoms belonging to an element can enter or leave a molecule of a chemical compound during a chemical reaction, but their total mass in the system will remain unchanged. Dalton assumed that hydrogen is the lightest element so he introduced the term relative atomic mass (Ar) as a ratio between the mass of an atom of the element and the mass of a hydrogen atom.(Table 2.)

| Simple Elements | |||||
|---|---|---|---|---|---|
| Fig. 1 | Hydrogen, its rel. weight | 1 | Fig. 11 | Strontites (SrO) | 46 |
| Fig. 2 | Azote | 5 | Fig. 12 | Barytes (BaO) | 68 |
| Fig. 3 | Carbone or charcoal | 5 | Fig. 13 | Iron | 38 |
| Fig. 4 | Oxygen | 7 | Fig. 14 | Zinc | 56 |
| Fig. 5 | Phosphorus | 9 | Fig. 15 | Copper | 56 |
| Fig. 6 | Sulphur | 13 | Fig. 16 | Lead | 95 |
| Fig. 7 | Magnesia (MgO) | 20 | Fig. 17 | Silver | 100 |
| Fig. 8 | Lime (CaO) | 23 | Fig. 18 | Platina | 100 |
| Fig. 9 | Soda (Na2CO3) | 28 | Fig. 19 | Gold | 140 |
| Fig. 10 | Potash (K2O) | 42 | Fig. 20 | Mercury | 167 |
| Binary, Ternary and Quaternary Compounds | |||||
| Fig. 21 | An atom of water, composed of 1 of oxygen and 1 of hydrogen | 8 | |||
| Fig. 22 | An atom of carbonic acid, 1 carbone + 2 oxygen | 19 | |||
| Fig. 23 | An atom of sulphuric acid, 1 sulphur + 3 oxygen | 34 | |||
Today's system of chemical symbols, based on the first letter (and eventually one more) of the Latin name of the element was introduced in 1813 by the Swedish chemist Jöns Jacob Berzelius (1779-1848). In 1818, after ten years of testing over 2000 compounds, he also published the atomic masses of elements which, for the time, were amazingly accurate. Berzelius considered oxygen a much more suitable unit than hydrogen, so he calculated the relative atomic masses of elements by setting the weight of oxygen to equal exactly 100.
| Namen. | Zeichen. | Gewicht. | Namen. | Zeichen. | Gewicht. |
| Oxygenium | O. | 100. | Palladium | Pl. | 1418. |
| Sulphuricum | S. | 201. | Hydrargyrum | Hg. | 2531.6 |
| Phosphoricum | P. | 167.512 | Argentum | Ag. | 2688.17 |
| Muriaticum | M. | 159.56 | Cuprum | Cu. | 806.45 |
| Fluoricum | F. | 60. | Niccolum | Ni. | 733.8 |
| Boracicum | B. | 73.27 | Cobaltum | Co. | 732.61 |
| Carbonicum | C. | 74.91 | Bismuthum | Bi. | 1774. |
| Nitricum | N. | 79.54 | Plumbum | Pb. | 2597.4 |
| Hydrogenium | H. | 6.636 | Stannum | Sn. | 1470.59 |
| Arsenicum | As. | 859.9 | Ferrum | Fe. | 693.64 |
| Molybdaenum | Mo. | 601.56 | Zincum | Zn. | 806.45 |
| Chromium | Ch. | 708.05 | Manganium | Mn. | 711.57 |
| Woframium | W. | 2424.24 | Uranium | U. | 3141.4 |
| Tellurium | Te. | 806.48 | Cerium | Ce. | 1148.8 |
| Stibium | Sb. | 1613. | Yttrium | Y. | 881.66 |
| Tantalum | Ta. | - | Beryllium | Be. | 683.3 |
| Titanium | Ti. | 1801? | Aluminium | Al. | 343. |
| Silicium | Si. | 304.35 | Magnesium | Ms. | 315.46 |
| Zirconium | Zr. | - | Calcium | Ca. | 510.2 |
| Osmium | Os. | - | Strontium | Sr. | 1418.14 |
| Iridium | I. | - | Barytium | Ba. | 1709.1 |
| Rhodium | R. | 1490.3 | Natrium | Na. | 579.32 |
| Platinum | Pt. | 1206.7 | Kalium | K. | 978.0 |
| Aurum | Au. | 2483.8 |
After Lavoisier the systematic approach and new experimental techniques soon led to the discovery of many new elements. It took less than fifty years after the publication of his book for the number of known elements to double. Two techniques had a significant role in this: electrolysis and the great reduction power of alkali metals was an excellent tool for isolating new elements, while atomic spectroscopy was responsible for their identification.
In October 1807 the British scientist Sir Humphry Davy (1778-1829) created the most powerful battery of his time by using 250 plates, which allowed him to conduct electricity through molten salts instead of aqueous solutions. Through electrolysis of molten potash (K2CO3), a mixture which has long been speculated to contain a new element, Davy successfully managed to obtain tiny beads of metallic potassium. The same week Davy produced a second alkali metal from caustic soda (NaOH) - sodium. When the beads of these metals were dropped in water they would catch fire and race across the surface with a high-pitched sound. The discovery of these highly reactive metals caused a lot of excitement, while the spectacular 'demonstrations' of these newfound elements often resulted in a couple of ladies from the audience fainting.
The German physicist Gustav Robert Kirchhoff (1824-1887), while studying colored vapors above substances heated to white glow, discovered that each element gives a unique and characteristic pattern of colored lines. Each element gives the same set of identifying lines, even when it's chemically combined with other elements. In 1859, together with the German chemist Robert Wilhelm Bunsen (1811-1899), he developed a spectroscope which turned out to be a supremely useful tool in the process of identifying new elements. By using this new research tool they themselves discovered two new elements, caesium (1860) and rubidium (1861). Kirchhoff and Bunsen also found that gasses absorb radiation of the same wavelength as the ones they emit. By studying the dark lines of the solar spectrum they discovered a few dark lines they speculated were from elements not yet found on Earth.
This abundance of new elements with an ever-widening range of properties soon began to raise many questions. How many elements are there exactly? Have they all been discovered? Are there possibly a myriad of elements? Some new elements, such as alkaline metals, had so many different properties that they could not be incorporated into any existing group of elements. Could there be some sort of fundamental law behind all of it?
The German chemist Johan Döbereiner (1780-1849) was the first in a line of chemists that recognized the relationship between atomic mass and chemical properties. He noticed that in a group of three elements with similar chemical properties the atomic mass of the second member of the "triad" was almost exactly in the middle of the atomic masses of the other two elements. He observed that the molar mass of strontium oxide was very close to the arithmetic mean of the molar masses of calcium oxide and barium oxide. The same regularity has been observed in the ratio of their specific weights. If sulphur, selenium, and tellurium belong to the same group, as was speculated, then the specific weight of selenium would be the arithmetic mean of the specific weights of sulphur and tellurium. According to his law the empirical atomic mass of selenium would be 32.239 + 129.243)/2 = 80.741 which is very close to the measured value found by Berzelius (78.383). By mid-century Döbereiner's 'triad law' was expanded to larger groups with four and five elements of similar properties in which there was a regular increase in the relative atomic mass. Döbereiner expressed his ideas as early as 1917, but published them only twelve years later when Berzelius's work on determining the relative atomic masses of bromine and iodine brought them into the spotlight. He observed that the newly discovered bromine element has chemical properties similar to those of chlorine and iodine. Not only that, but its atomic mass was approximately the arithmetic mean of the atomic masses of these two elements (35.470 + 126.470)/2 = 80.470 (the empirically found atomic value for selenium is 79.263).
| a) Salt-forming elements | b) Acid-forming elements | c) Alkali-forming elements | d) Alkaline-earth-forming elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 221.325 | Cl | 201.165 | S | 95.310 | L | 256.019 | Ca | ||||
| 789.145 | I | 806.452 | Te | 489.916 | K | 856.880 | Ba | ||||
|
1010.470
/
2
| Br |
1007.617
/
2
| Se |
585.226
/
2
| Na |
1112.899
/
2
| Sr | ||||
The First International Congress of Chemists was convened on September 1860 in Karsruhe, Germany. Almost all notable chemists from across the entirety of Europe, as well as many whose name was not yet known, attended this event. The very future of chemistry depended on the results of this congress. The spirited and charismatic Itallian chemist Stanislao Cannizzaro (1826-1910) started the atomic weight discussion. He claimed that Avogardo's hypothesis led directly to atomic masses of gaseous elements, and from there to the atomic masses of other elements. The Itallian physicist Amedeo Avogadro (1776-1856) proposed two hypotheses in 1811: (1) the smallest particles of a gas are groups of atoms - molecules; (2) equal volumes of different gasses, at the same pressure and temperature, contain the same number of molecules. If we know that the volume of oxygen is 16 times heavier than the same volume of hydrogen and if the atomic mass of hydrogen is 1, the atomic mass of oxygen must be 16. Since only half of a volume of oxygen combines with one volume of hydrogen to form water, logic dictates that the formula of water is H 2O, and not HO. Cannizzaro's ideas were quickly accepted, and copies of his table of the atomic weights all eagerly snapped up.
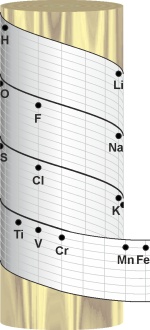
With the intention of checking whether there is any actual regularity among the elements the French geologist Alexandre Emile Becuyer De Chancourtois (1820-1886) published an article in 1862 describing his 'Vis Tellurique'. De Chancourtois divided the paper tape into equal parts by using the atomic weight of hydrogen as the base unit, and then sorted the elements on it based on the new atomic weights Cannizzaro provided. He would then wind the tape into a helix on cylinders with different diameters. When he wound the tape onto a cylinder whose base circumference equaled 16 units of atomic mass he observed that elements placed vertically one above another had similar properties. Unfortunately, the article was very difficult to understand because de Chancourtois used geological names, and due to error from the editor it was published without diagrams.
The English chemist John Newlands (1837-1898) arranged all of the elements known at the time into a table in order of relative atomic mass, starting with hydrogen and ending with thorium. When he arranged the elements by their equivalents (atomic masses shown as multiples of the number eight), with a couple of minor exceptions, he noticed that elements belonging to the same group often appeared in the same row (Table 4.). He also noticed that the numbers in matching elements generally differed either by 7, or by a multiple of 7. As such in the nitrogen group there are 7 elements between nitrogen and phosphorus; 14 elements between arsenic and phosphorus; 14 elements between arsenic and tin, and 14 elements between tin and bismuth as well. Newlands called his relationship the 'Law of Octaves', comparing the elements to the notes in a musical scale. In this grid-based distribution sodium found itself next to a very similar potassium, and magnesium next to the equally similar calcium. When Newlands expanded the table to include all known elements he found that halogens chlorine, bromine, and iodine, all appeared in the same horizontal row. The properties of some elements, especially of those with larger atomic masses, simply did not fit. However, even still, Newland's Law of Octaves was undoubtedly a step forward when compared to all previous methods. He presented his discoveries in 1865 to the Chemical Society in London, but the gathered dignitaries simply laughed at his Law of Octaves and sarcastically advised him to try to sort the elements by alphabetical order.
| No. | No. | No. | No. | No. | No. | No. | No. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | 1 | F | 8 | Cl | 15 | Co & Ni | 22 | Br | 29 | Pd | 36 | I | 42 | Pt & Ir | 50 |
| Li | 2 | Na | 9 | K | 16 | Cu | 23 | Rb | 30 | Ag | 37 | Cs | 44 | Os | 51 |
| G | 3 | Mg | 10 | Ca | 17 | Zn | 24 | Sr | 31 | Cd | 38 | Ba & V | 45 | Hg | 52 |
| Bo | 4 | Al | 11 | Cr | 19 | Y | 25 | Ce & La | 33 | U | 40 | Ta | 46 | Tl | 53 |
| C | 5 | Si | 12 | Ti | 18 | In | 26 | Zr | 32 | Sn | 39 | W | 47 | Pb | 54 |
| N | 6 | P | 13 | Mn | 20 | As | 27 | Di & Mo | 34 | Sb | 41 | Nb | 48 | Bi | 55 |
| O | 7 | S | 14 | Fe | 21 | Se | 28 | Ro & Ru | 35 | Te | 43 | Au | 49 | Th | 56 |
(Elements that have the same equivalent are labeled with the same number)
Döbereiner noticed similarities between isolated groups of elements. De Chancourtois recognized a certain pattern in the repetition of properties. Newlands expanded this regularity, but his Law of Octaves only worked for the first 16 elements. This was partly due to the mistakes while calculating the various atomic masses, and partly because Newlands didn't leave any room for still undiscovered elements.
By the middle of the century it became increasingly clear that there is some periodicity in the layout of the elements. Cooke, Cremers, Gladstone, Gmelin, Lenssen, Pettenkofer, and especially Dumas already discovered numerous facts that contributed to that opinion. It seemed that even a single glance at the elements was enough to explain the law by which they were sorted. The elements could be sorted by their ascending atomic weights, or they could be gathered into groups with similar properties, but the overall layout of the elements remained elusive.
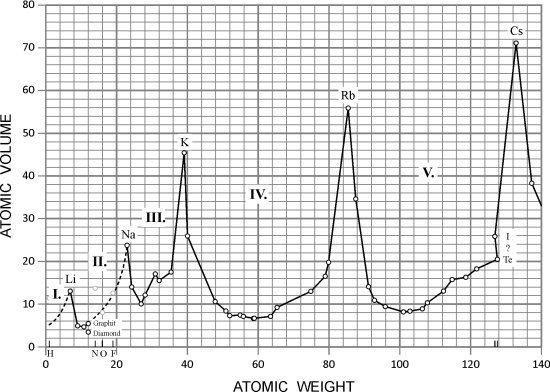
The German chemist Julius Lothar Meyer (1830-1895) found that the physical properties of elementary substances (atomic volume, melting point, boiling point, density, etc.) are periodic functions of the relative atomic mass. Meyer was strongly influenced by Cannizzar's ideas, and in 1864, he presented his ideas about the relationship between physical properties of elements and their atomic mass in his textbook "Die Modernen Theorien der Chemie". In it Meyer gave a table with twenty eight elements ordered by their atomic weight in six families, and as a link between family members he took valence.
Meyer noticed that one of the properties that periodically changed with the atomic mass is the element's atomic volume. By putting the relative atomic masses on the abscissa, and the atomic volumes as the ordinate, he obtained an interrupted curve with particularly sharply expressed breaks at the relative atomic masses of alkali metals (Figure 3.) Based on these discoveries he assembled a periodic table that greatly resembled the one we know today and in which hydrogen had a special place. Unfortunately, he published his table (Table 5.) only in 1870.
| I. | II. | III. | IV. | V. | VI. | VII. | VIII. | IX. |
|---|---|---|---|---|---|---|---|---|
| B=11.0 | Al=27.3 | -- | ?In=113.4 | Tl=202.7 | ||||
| -- | -- | -- | ||||||
| C=11.97 | Si =28 | -- | Sn=117.8 | Pb=206.4 | ||||
| Ti=48 | Zr=89.7 | -- | ||||||
| N=14.01 | P=30.9 | As=74.9 | Sb=122.1 | Bi=207.5 | ||||
| V=51.2 | Nb=93.7 | Ta=182.2 | ||||||
| O=15.96 | S=31.98 | Se=78 | Te=128? | -- | ||||
| Cr=52.4 | Mo=95.6 | W=183.5 | ||||||
| - | F=19.1 | Cl=35.38 | Br=79.75 | I=126.5 | -- | |||
| Mn=54.8 | Ru=103.5 | Os=198.6? | ||||||
| Fe=55.9 | Rh=104.1 | Ir=196.7 | ||||||
| Co = Ni = 58.6 | Pd=106.2 | Pt=196.7 | ||||||
| Li=7.01 | Na=22.99 | K=39.04 | Rb=85.2 | Cs=132.7 | -- | |||
| Ag=107.66 | Au=196.2 | |||||||
| ?Be=9.8 | Mg=23.9 | Ca=39.9 | Sr=87.0 | Ba=136.8 | -- | |||
| Zn=64.9 | Cd=111.6 | Hg=199.8 | ||||||
Meyer's table contained only 54 elements because he did not include elements whose data he doubted and those that could not fit into his table. As such his table was missing, besides hydrogen, all lanathides and actinoids that were well known at the time (Y, Eb, (Tb?), Ce, La, Di, Th, U). He assumed that these elements, or some currently unknown ones, would fill out the gaps in the table, and that due to the additions or future discoveries certain elements would need to change places.
In 1867 Dmitry Ivanovič Mendeljejev (1834-1907) was appointed as the professor of General Chemistry at the University of St. Petersburg. Since he could not find a textbook that satisfied his needs, he began writing his own. The result of this was the classic "The Principles of Chemistry" textbook that took from 1868 to 1870 to be fully finished. During the writing of his book Mendeleyev was examining in detail the relationship between the properties of elements in order to create a system to classify them. It was necessary to find some basic principle by which the elements could be ordered because the structure of the entire book depended on it. By that point it was known that there are sixty-three chemical elements, which each of elements contained different atoms, and that atoms of each element had their own unique properties. However, some elements contained similar properties which allowed them to be gathered together into groups.
Mendeleyev linked together the element problem and his favorite card game - solitaire. He put the names of elements onto empty cards, adding next to them their atomic masses and chemical properties. He hoped that some elements would fit together like cards: by groups of similar properties (such as card colors) in which the elements would be ordered by their atomic masses (imitating the numerical sequence in cards). In his head Mendeleyev saw the elements ordered by their atomic weights and their properties repeating in periodic intervals. This table and its accompanying remarks, under the title of "On the Correlation between the Properties of the Elements and their Atomic Weights ", were first presented to the Russian Chemical Society on March 1, 1869. The article was soon published in the first volume of the society's new journal.
| Ti=50 | Zr=90 | ?=180 | |||
| V=51 | Nb=94 | Ta=182 | |||
| Cr=52 | Mo=96 | W=186 | |||
| Mn=55 | Rh=104.4 | Pt=197.4 | |||
| Fe=56 | Ru=104.4 | Ir=198 | |||
| Ni=Co=59 | Pd=106.6 | Os=199 | |||
| H=1 | Cu=63.4 | Ag=108 | Hg=200 | ||
| Be=9.4 | Mg=24 | Zn=65.2 | Cd=112 | ||
| B=11 | Al=27,4 | ?=68 | Ur=116 | Au=197? | |
| C=12 | Si=28 | ?=70 | Sn=118 | ||
| N=14 | P=31 | As=75 | Sb=122 | Bi=210? | |
| O=16 | S=32 | Se=79.4 | Te=128? | ||
| F=19 | Cl=35.5 | Br=80 | J=127 | ||
| Li=7 | Na=23 | K=39 | Rb=85.4 | Cs=133 | Tl=204 |
| Ca=40 | Sr=87.6 | Ba=137 | Pb=207 | ||
| ?=45 | Ce=92 | ||||
| ?Er=56 | La=94 | ||||
| ?Yt=60 | Di=95 | ||||
| ?In=75.6 | Th=118? |
In the assumed system the atomic weight of the element is unique and it serves as the basis for deciding the position of the element. The comparison between previously known groups of elements by the weight of their atoms lead to the conclusion that the distribution of elements by their atomic weights does not disturb the natural similarities existing between the elements but, on the contrary, directly points to them.
Mendeleyev himself admitted that there were some inconsistencies in this scheme. In cases where the atomic masses didn't accurately follow the ascending order Mendeleyev suspected the atomic weight of the element, suggesting that it was incorrectly calculated. In places where no element fit the scheme he left an empty space, predicting that the void will eventually be filled with elements that have not yet been discovered. There were also some areas where it appeared that the chemical properties did not follow the pattern, or where the elements had to be repositioned. Despite these obvious anomalies Mendeljev felt he was in the right. He was deeply convinced that these irregularities could be explained and that in his periodic law, as he called it, there must be an answer.
Two years later, in 1871, Mendeleyev presented a new table of the periodic system in order to show the periodic law in detail. He was so certain in his approach that he claimed it was possible to predict the properties of three still undiscovered elements, and for each of the three new elements (eka-aluminium, eka-boron, and eka-silicon) he suggested possible properties including density, radius, and relationship when combining with oxygen. The scientific world was confused, and many were mocking Mendeleyev's prediction. Moreover, the years that followed were surprisingly bleak when it came to discovering new elements.
R e i h e n |
Gruppe I. – R2O |
Gruppe II. – RO |
Gruppe III. – R2O3 |
Gruppe IV. RH4 RO2 |
Gruppe V. RH3 R2O5 |
Gruppe VI. RH2 RO3 |
Gruppe VII. RH R2O7 |
Gruppe VIII. – RO4 |
|---|---|---|---|---|---|---|---|---|
| 1 | H=1 | |||||||
| 2 | Li=7 | Be=9,4 | B=11 | C=12 | N=14 | O=16 | F=19 | |
| 3 | Na=23 | Mg=24 | Al=27,3 | Si=28 | P=31 | S=32 | Cl=35,5 | |
| 4 | K=39 | Ca=40 | – =44 | Ti=48 | V=51 | Cr=52 | Mn=55 | Fe=56, Co=59,
Ni=59, Cu=63 |
| 5 | (Cu=63) | Zn=65 | – =68 | – =72 | As=75 | Se=78 | Br=80 | |
| 6 | Rb=85 | Sr=87 | ?Yt=88 | Zr=90 | Nb=94 | Mo=96 | – =100 | Ru=104, Rh=104,
Pd=106, Ag=108 |
| 7 | (Ag=108) | Cd=112 | In=113 | Sn=118 | Sb=122 | Te=125 | J=127 | |
| 8 | Cs=133 | Ba=137 | ?Di=138 | ?Ce=140 | – | – | – | – – – – |
| 9 | (–) | – | – | – | – | – | – | |
| 10 | – | – | ?Er=178 | ?La=180 | Ta=182 | W=184 | – | Os=195, Ir=197,
Pt=198, Au=199 |
| 11 | (Au=199) | Hg=200 | Tl=204 | Pb=207 | Bi=208 | – | – | |
| 12 | – | – | – | Th=231 | – | U=240 | – | – – – – |
Then, in the late summer of 1875, the French chemist Paul Lecoq de Boisbaudran discovered a new element in a zinc sulfide sample. The properties of the newly discovered element, called gallium by the Latin name for France, were astonishingly similar to eka-aluminium. This was not purely a coincidence, as was demonstrated in 1879 by Nilson's discovery of scandium with properties Mendeley predicted for eka-borone. These discoveries, which were the confirmation for his predictions and evidence for his law, brought Mendeley to the very top of the scientific world. In 1882 the Royal Society of England awarded Mendeley and Meyer with a Davy medal. A few years later, in 1886, the German chemist Clemens Winkler discovered a new element, germanium, whose properties matched those Mendeleyev predicted for eka-silicon during a routine analysis of the mineral argirodite.
Meyer and Mendeley were among the chemists that attended the Congress in Karlsruhe in 1860, and were heavily impressed by Cannizzar's presentation of Avogadro's hypotheses. For both of the incentive to develop a periodic table was their desire to write a comprehensive textbook - a way to present more than sixty known elements to students. By using similar rows as Mendeley, Meyer managed to find almost the same pattern among the elements at same time as Medeljev. Why then was it so difficult to create a periodic table? The problem was that the chemists in the 19th century were arranging elements according to the wrong 'law': the properties of elements were not a periodic function of their atomic masses, but rather their atomic numbers. This is where Mendeljejev's intuition and the ability to spot patterns in seemingly unrelated data came into effect. He envisioned a place for each element in the table, and even facts were not enough to muddy that image. As such he later noted: "Although there are doubts surrounding some unresolved places, I have not once suspected the universality of this law ".
In 1894, the British scientists Lord Rayleigh (1842-1919) and Sir William Ramsay (1852-1916) isolated a new element from the air (argon) for which there was no place in the periodic system. The new element seemed to be in conflict with the already established knowledge about the elements. It was only the isolation of helium from uranium ore clevite in 1895 that shed new light on this problem. In 1897 Ramsay suggested the existence of a group of inert gasses, and within it he predicted empty spaces for the still undiscovered elements. By the end of the century Ramsay and his associates isolated the rest of the noble gasses - neon, krypton, and xenon. What is interesting is that all three, Meyer, Mendeley, and Ramsay, studied at Heidelberg with Robert Bunsen, though at different times.
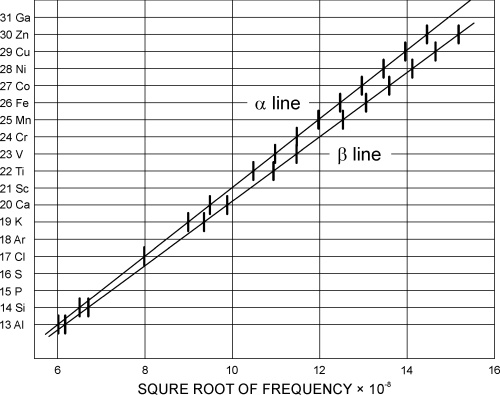
Almost fifty years after Mendeley, in 1913, the British chemist Henrik Moseley (1887-1915) published the results of his measurements of the X-ray wavelengths obtained from anti-cathodes of various elements. He noticed that the X-ray wavelengths regularly changed in the row of elements with increasing atomic masses. Mosley assigned each element in the row a number which he called an atomic number of the element (Z), and instead of sorting the periodic table by atomic weights he did it by atomic numbers.
After the discovery of plutonium (1940) the American physicist Glenn Theodore Seaborg (1912-1999) presented in 1944 his "actinide concept", which became the foundation for many important discoveries in the research of heavy elements. This concept predicted that the fourteen actinoids, including the first eleven transuranium elements, would form a group of transitional elements such as lanthanide. The predictions for the chemical properties and order of these heavy elements helped Seaborg and his colleagues add ten new elements to the periodic system: plutonium, americium, curium, berkelium, californium, einsteinium, fermium, mendelevium, nobelium and seaborgium.
The periodic law revealed important analogies between the elements and inspired an interest in inorganic chemistry that remains strong to this very day through the creation of artificial, short-lived elements in modern accelerators. More than 700 different shapes of the periodic system of elements, in 146 different types and sub-types, were submitted since Mendeljejev's first paper.
Bibliografía:
- I. Filipović, S. Lipanović: Opća i anorganska kemija, Školska knjiga, Zagreb, 1982.
- J. Emsley, The Elements, Oxford University Press, New York, 2000.
- P. Strathern, Mandeleyev's Dream: The Quest for the Elements, Thomas Dune Books, New York, 2000.
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Butterworth-Heinemann, Oxford, 2001.
- "Encyclopedia Britannica 2004 Ultimate Reference Suite DVD". Encyclopedia Britannica Inc. 2004.
- Rod Beavon. "Periodicity." 21 Dec. 2002. <http://www.rod.beavon.clara.net/periodic.htm>.
- "Selected Classic Papers from the History of Chemistry", 5 Dec. 2002. <http://webserver.lemoyne.edu/giunta/papers.html>.
- "From Alchemy to Chemistry: Five Hundred Years of Rare and Interesting Books". University of Illinois. 11 Apr. 2010. <http://www.scs.uiuc.edu/~mainzv/exhibit/>.
- Boyle, Robert. "The sceptical chymist." J.M. Dent & Sons, ltd. London, 1911. Retrieved 16 July 2017 via <HathiTrust Digital Library>.
- Lavoisier, Antoine-Laurent de. "Traité Élémentaire de Chimie." Cuchet, Paris, 1793. Retrieved 19 July 2017 via <Bibliothèque nationale de France>.
- Berzelius, Jöns Jacob. "Neues System der Mineralogie." Schrag. Nürnberg, 1816. Retrieved 23 July 2017 via <Bayerische Staatsbibliothek>.
- Döbereiner, Johan. Annalen der Physik und Chemie 2 Folge: Bd. 15-16. p. 301-307. Leipzig. 1829. Retrieved 3 Aug. 2017 via <HathiTrust Digital Library>.
- Cannizzaro, Stanislao. "Sketch of a course of chemical philosophy." Alembic Club. Edinburgh, 1858. Retrieved 28 July 2017 via <Internet Archive>.
- Chancourtois, A. E. Becuyer de. "A first foreshadowing of the periodic law." Nature. p. 186-188. London, 1889. Retrieved 28 July 2017 via <Dolnośląska Digital Library>.
- Meyer, Julius Lothar. "Die Natur der chemischen Elemente als Function ihrer Atomgewichte." Annalen der Chemie. Supplementband 7. 354. 1870. Retrieved 17 July 2017 via <Selected Classic Papers from Le Moyne College>.
Citación de esta página:
Generalic, Eni. "History of the Periodic table of elements." EniG. Tabla periódica de los elementos. KTF-Split, 13 Feb. 2025. Web. 8 Apr. 2025. <https://www.periodni.com/es/history_of_periodic_table_of_elements.html>.
Tablas y artículos
- Tabla periódica
- Calculadoras online
- Calculadora científica para química
- Calculadora con leyes de los gases
- Calculadora de masa molar
- Convertir ángulo
- Convertidor números romanos
- Sistema de numeración convertidor
- Preparación de las soluciones
- Etiquetado de envases químicos
- Calculadora de números de oxidacion
- ARS metodo
- Método del número de oxidación
- Método del ion-electrón
- Método de eliminación de Gauss
- Juego de memoria
- Encuentre los pares
- Tablas y artículos
- Química
- Lista de acrónimos y abreviaturas
- Sistemas cristalinos y redes de Bravais
- SGA - Pictogramas de peligro
- Diamante de peligro de NFPA 704
- Constantes físicas fundamentales
- Constantes del producto de solubilidad
- SI - Sistema Internacional de Unidades
- Composición de mezclas y soluciones
- Cálculo estequiométrico
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Ecología
- Diseño web
- Diccionario de química (inglés-croata)
- Química
- Descargas
- ≡ Menú
