KRYPTON
NOBLE GAS
| Atomic number: | 36 |
| Group numbers: | 18 |
| Period: | 4 |
| Electronic configuration: | [Ar] 3d10 4s2 4p6 |
| Formal oxidation number: | 0 |
| Electronegativities: | - |
| Atomic radius / pm: | 202 |
| Relative atomic mass: | 83.798(2) |
Krypton was discovered by Sir William Ramsay and Morris William Travers (GB) in 1898. The origin of the name comes from the Greek word kryptos meaning hidden. It is a colourless, odourless rare noble gas that reacts only with fluorine. Krypton is obtained from production of liquid air. Krypton is used in lighting products. It is used as inert filler-gas in incandescent bulbs and some is mixed with argon in fluorescent lamps. The most important use is in flashing stroboscopic lamps that outline airport runways. The price of 99.995 % pure krypton gas costs 165.30 €/dm3 in small quantities (1 dm3) and about 4.51 €/dm3 in large quantities (300 dm3).
| Density / g dm-3: | 2823 | (solid, m.p.) |
| 2413 | (liquid, b.p.) | |
| 3.7493 | (gas, 273 K) | |
| Molar volume / cm3mol-1: | 29.68 | (solid, m.p.) |
| 34.73 | (liquid, b.p.) | |
| 22350.84 | (gas, 273 K) | |
| Electrical resistivity / µΩcm: | - | (20 °C) |
| Thermal conductivity / W m-1K-1: | 0.0095 |
| Melting point / °C: | -157.36 |
| Boiling point / °C: | -153.22 |
| Heat of fusion / kJ mol-1: | 1.64 |
| Heat of vaporization / kJ mol-1: | 9.05 |
| Heat of atomization / kJ mol-1: | 0 |
| First ionization energy / kJ mol-1: | 1350.77 |
| Second ionization energy / kJ mol-1: | 2350.39 |
| Third ionization energy / kJ mol-1: | 3565.16 |
| in the atmosphere / ppm: | 1.14 |
| in the Earth's crust / ppm: | 0.00001 |
| in the oceans / ppm: | 0.0003 |
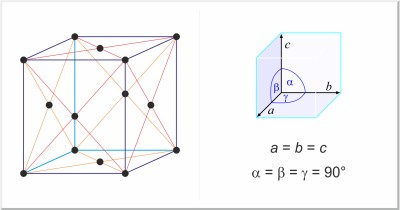
| Crystal structure: | face-centered cubic |
| Unit-cell dimensions / pm: | a=572.1 |
| Space group: | Fm3m |
| Isotope | Relative atomic mass | Mass percent (%) |
|---|---|---|
| 78Kr | 77.920386(7) | 0.35(2) |
| 80Kr | 79.916378(4) | 2.25(2) |
| 82Kr | 81.913485(3) | 11.6(1) |
| 83Kr | 82.914136(3) | 11.5(1) |
| 84Kr | 83.911507(3) | 57.0(3) |
| 86Kr | 85.910610(1) | 17.3(2) |
| Balanced half-reaction | Eo / V | |
|---|---|---|
| 35 Bromine | ← | 36 Krypton | → | 37 Rubidium |
Citing this page:
Generalic, Eni. "Krypton." EniG. Periodic Table of the Elements. KTF-Split, 13 Feb. 2025. Web. {Date of access}. <https://www.periodni.com/kr.html>.
Articles and tables
- Periodic table
- Online calculators
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Preparation of solutions
- Labeling of chemical containers
- Oxidation numbers calculator
- ARS method
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Memory game
- Find the pairs
- Articles and tables
- Chemistry
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Ecology
- Web design
- Chemistry dictionary
- Chemistry
- Downloads
- ≡ Menu
