SULFUR
CHALCOGENS ELEMENT
| Atomic number: | 16 |
| Group numbers: | 16 |
| Period: | 3 |
| Electronic configuration: | [Ne] 3s2 3p4 |
| Formal oxidation number: | -2 +4 +6 |
| Electronegativities: | 2.58 |
| Atomic radius / pm: | 103.5 |
| Relative atomic mass: | [32.059, 32.076] |
Sulfur has been known since ancient times. The origin of the name comes from the Sanskrit word sulvere meaning sulfur; also from the Latin word sulphurium meaning sulfur. It is a pale yellow, odourless, brittle solid, which is insoluble in water but soluble in carbon disulfide. Sulfur is found in pure form and in ores like cinnabar, galena, sphalerite and stibnite. Pure form is obtained from underground deposits by the Frasch process. Sulfur is used in matches, gunpowder, medicines, rubber and pesticides, dyes and insecticides, and for making sulfuric acid (H2SO4). The price of 99.5 % pure sulfur powder is 23.60 € for 500 g.
| Density / g dm-3: | 2070 | (alpha, 293 K) |
| 1957 | (beta, 293 K) | |
| 1891 | (liquid, 393 K) | |
| Molar volume / cm3mol-1: | 15.49 | (alpha, 293 K) |
| 16.38 | (beta, 293 K) | |
| 16.96 | (liquid, 393 K) | |
| Electrical resistivity / µΩcm: | 2E+23 | (20 °C) |
| Thermal conductivity / W m-1K-1: | 0.269 |
| Melting point / °C: | 115.21 |
| Boiling point / °C: | 444.60 |
| Heat of fusion / kJ mol-1: | 1.7175 |
| Heat of vaporization / kJ mol-1: | 9.62 |
| Heat of atomization / kJ mol-1: | 276.6 |
| First ionization energy / kJ mol-1: | 999.60 |
| Second ionization energy / kJ mol-1: | 2251.78 |
| Third ionization energy / kJ mol-1: | 3356.75 |
| in the atmosphere / ppm: | 1 |
| in the Earth's crust / ppm: | 260 |
| in the oceans / ppm: | 900 |
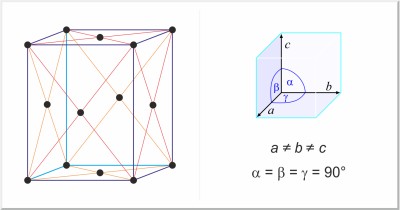
| Crystal structure: | face-centered orthorhombic |
| Unit-cell dimensions / pm: | a=1046.46, b=1286.60, c=2448.60 |
| Space group: | Fddd |
| Isotope | Relative atomic mass | Mass percent (%) |
|---|---|---|
| 32S | 31.9720707(1) | 95.02(9) |
| 33S | 32.9714585(1) | 0.75(4) |
| 34S | 33.9678668(1) | 4.21(8) |
| 36S | 35.9670809(3) | 0.02(1) |
| Balanced half-reaction | Eo / V | |
|---|---|---|
| S(s) + 2e- → S2- | - 0.476 | |
| S(s) + H+ + 2e- → HS- | - 0.065 | |
| S(s) + 2H+ + 2e- → H2S(g) | +0.141 | |
| SO42- + 4H+ + 2e- → H2SO3 + H2O | +0.172 | |
| SO42- + 4H2O + 2e- → SO3- + 2OH- | - 0.93 | |
| SO42- + 8H+ + 6e- → S(s) + 4H2O | +0.357 | |
| HSO4- + 7H+ + 6e- → S(s) + 4H2O | +0.339 | |
| SO42- + 8H+ + 8e- → S2- + 4H2O | +0.149 | |
| SO42- + 9H+ + 8e- → HS- + 4H2O | +0.252 | |
| SO42- + 10H+ + 8e- → H2S(g) + 4H2O | +0.303 | |
| HSO4- + 9H+ + 8e- → H2S(g) + 4H2O | +0.289 | |
| S2O82- + 2e- → 2SO42- | +2.01 | |
| S2O82- + 2H+ + 2e- → 2HSO4- | +2.123 | |
| S4O62- + 2e- → 2S2O32- | +0.08 | |
| S4O62- + 12H+ + 10e- → 4S(s) + 6H2O | +0.416 | |
| S2O62- + 2e- → 2SO32- | +0.026 | |
| SO2(g) + 4H+ + 4e- → S(s) + 2H2O | +0.451 | |
| H2SO3 + 4H+ + 4e- → S(s) + 3H2O | +0.450 | |
| 2SO32- + 6H+ + 4e- → S2O32- + 3H2O | +0.705 | |
| 2HSO3- + 4H+ + 4e- → S2O32- + 3H2O | +0.491 | |
| 4HSO3- + 8H+ + 6e- → S4O62- + 6H2O | +0.581 | |
| 4H2SO3 + 4H+ + 6e- → S4O62- + 6H2O | +0.509 | |
| SO32- + 6H+ + 6e- → S2- + 3H2O | +0.231 | |
| S22- + 2H+ + 2e- → 2HS- | +0.298 | |
| S22- + 2e- → 2S2- | - 0.524 | |
| S32- + 3H+ + 4e- → 3HS- | +0.097 | |
| S42- + 4H+ + 6e- → 4HS- | +0.033 | |
| S52- + 5H+ + 8e- → 5HS- | +0.003 |
| 15 Phosphorus | ← | 16 Sulphur | → | 17 Chlorine |
Citing this page:
Generalic, Eni. "Sulfur." EniG. Periodic Table of the Elements. KTF-Split, 13 Feb. 2025. Web. {Date of access}. <https://www.periodni.com/s.html>.
Articles and tables
- Periodic table
- Online calculators
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Preparation of solutions
- Labeling of chemical containers
- Oxidation numbers calculator
- ARS method
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Memory game
- Find the pairs
- Articles and tables
- Chemistry
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Ecology
- Web design
- Chemistry dictionary
- Chemistry
- Downloads
- ≡ Menu
