SCANDIUM
TRANSITION ELEMENT: SCANDIUM GROUP
| Atomic number: | 21 |
| Group numbers: | 3 |
| Period: | 4 |
| Electronic configuration: | [Ar] 3d1 4s2 |
| Formal oxidation number: | +3 |
| Electronegativities: | 1.36 |
| Atomic radius / pm: | 160.6 |
| Relative atomic mass: | 44.955 908(5) |
Scandium was discovered by Lars Fredrik Nilson (SE) in 1879. The origin of the name comes from the Latin word Scandia meaning Scandinavia. It is a fairly soft, silvery-white metal. It burns easily and tarnishes readily in air, and react with water to form hydrogen gas Scandium occurs mainly in the minerals thortveitile (~34% scandium), wiikite and in some tin and tungsten ores. Pure scandium is obtained as a by-product of uranium refining. Scandium metal is used in some aerospace applications. Scandium oxide (Sc2O3) is used in the manufacture of high-intensity electric lamps. Scandium iodide (ScI3) is used in lamps that produce light having a colour closely matching natural sunlight. The price of 99.9 % pure scandium ingot is 263.10 € for 1 g.
| Density / g dm-3: | 2989 | (273 K) |
| Molar volume / cm3mol-1: | 15.04 | (273 K) |
| Electrical resistivity / µΩcm: | 61 | (20 °C) |
| Thermal conductivity / W m-1K-1: | 15.8 |
| Melting point / °C: | 1541 |
| Boiling point / °C: | 2836 |
| Heat of fusion / kJ mol-1: | 15.9 |
| Heat of vaporization / kJ mol-1: | 376.1 |
| Heat of atomization / kJ mol-1: | 376.02 |
| First ionization energy / kJ mol-1: | 633.09 |
| Second ionization energy / kJ mol-1: | 1234.99 |
| Third ionization energy / kJ mol-1: | 2388.67 |
| in the atmosphere / ppm: | - |
| in the Earth's crust / ppm: | 30 |
| in the oceans / ppm: | 0.00004 |
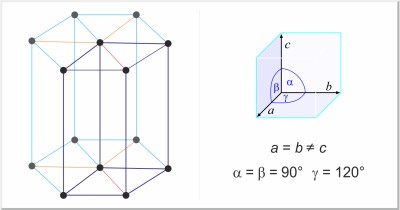
| Crystal structure: | hexagonal |
| Unit-cell dimensions / pm: | a=330.90, c=527.3 |
| Space group: | P63/mmc |
| Isotope | Relative atomic mass | Mass percent (%) |
|---|---|---|
| 45Sc | 44.955910(1) | 100 |
| Balanced half-reaction | Eo / V | |
|---|---|---|
| Sc3+ + 3e- → Sc(s) | - 2.08 |
| 20 Calcium | ← | 21 Scandium | → | 22 Titanium |
Citing this page:
Generalic, Eni. "Scandium." EniG. Periodic Table of the Elements. KTF-Split, 13 Feb. 2025. Web. 24 Apr. 2025. <https://www.periodni.com/sc.html>.
Articles and tables
- Periodic table
- Online calculators
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Preparation of solutions
- Labeling of chemical containers
- Oxidation numbers calculator
- ARS method
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Memory game
- Find the pairs
- Articles and tables
- Chemistry
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Ecology
- Web design
- Chemistry dictionary
- Chemistry
- Downloads
- ≡ Menu
