Np
Neptunium
NEPTUNIUM
TRANSITION ELEMENT: ACTINIDE
| Atomic number: | 93 |
| Group numbers: | 3 |
| Period: | 7 |
| Electronic configuration: | [Rn] 5f4 6d1 7s2 |
| Formal oxidation number: | +3 +4 +5 +6 |
| Electronegativities: | 1.3 |
| Atomic radius / pm: | 130 |
| Relative atomic mass: | - |
Neptunium was discovered by Edwin Mattison McMillan and Philip Hauge Abelson (US) in 1940. Named after the planet Neptune. It is a rare, silvery radioactive, radiotoxic metal that resists alkalis, reacts with oxygen, acid and steam. Neptunium was produced by bombarding uranium with slow neutrons.
| Density / g dm-3: | 20250 | (293 K) |
| Molar volume / cm3mol-1: | 11.70 | (293 K) |
| Electrical resistivity / µΩcm: | 122 | (20 °C) |
| Thermal conductivity / W m-1K-1: | 6.3 |
| Melting point / °C: | 644 |
| Boiling point / °C: | 3902 |
| Heat of fusion / kJ mol-1: | 9.46 |
| Heat of vaporization / kJ mol-1: | 336.6 |
| Heat of atomization / kJ mol-1: | 457 |
| First ionization energy / kJ mol-1: | 604.55 |
| Second ionization energy / kJ mol-1: | - |
| Third ionization energy / kJ mol-1: | - |
| in the atmosphere / ppm: | - |
| in the Earth's crust / ppm: | - |
| in the oceans / ppm: | - |
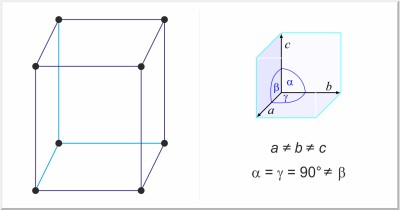
| Crystal structure: | simple monoclinic |
| Unit-cell dimensions / pm: | a=472.3, b=488.7, c=666.3 |
| Space group: | Pmcn |
| Isotope | Relative atomic mass | Mass percent (%) |
|---|---|---|
| 236Np | 233.039 64(2) | * |
| 237Np | 237.048 17(2) | * |
| Balanced half-reaction | Eo / V | |
|---|---|---|
| 92 Uranium | ← | 93 Neptunium | → | 94 Plutonium |
Citing this page:
Generalic, Eni. "Neptunium." EniG. Periodic Table of the Elements. KTF-Split, 18 Jan. 2024. Web. {Date of access}. <https://www.periodni.com/np.html>.
Articles and tables
- Periodic table
- Online calculators
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Preparation of solutions
- Labeling of chemical containers
- Oxidation numbers calculator
- ARS method
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Memory game
- Find the pairs
- Articles and tables
- Chemistry
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Ecology
- Web design
- Chemistry dictionary
- Chemistry
- Downloads
- ≡ Menu
Copyright © 1998-2024 by Eni Generalic. All rights reserved. | Bibliography | Disclaimer
