U
Uranium
URANIUM
TRANSITION ELEMENT: ACTINIDE
| Atomic number: | 92 |
| Group numbers: | 3 |
| Period: | 7 |
| Electronic configuration: | [Rn] 5f3 6d1 7s2 |
| Formal oxidation number: | +3 +4 +5 +6 |
| Electronegativities: | 1.7 |
| Atomic radius / pm: | 138.5 |
| Relative atomic mass: | 238.028 91(3) |
Uranium was discovered by Martin Heinrich Klaproth (DE) in 1789. Named after the planet Uranus. It is a silvery-white, dense, ductile, malleable, radioactive, radiotoxic metal that resists alkalis, tarnishes in air and reacts with steam and acids. Uranium occurs in many rocks, but in large amounts only in such minerals as pitchblende and carnotite. For many centuries it was used as a pigment for glass. Now it is used as a fuel in nuclear reactors and in bombs. The price of 99.7 % pure uranium turnings is 217.60 € for 25 g.
| Density / g dm-3: | 18950 | (293 K) |
| 17907 | (m.p.) | |
| Molar volume / cm3mol-1: | 12.56 | (293 K) |
| 13.29 | (m.p.) | |
| Electrical resistivity / µΩcm: | 30 | (20 °C) |
| Thermal conductivity / W m-1K-1: | 27.6 |
| Melting point / °C: | 1135 |
| Boiling point / °C: | 4131 |
| Heat of fusion / kJ mol-1: | 15.5 |
| Heat of vaporization / kJ mol-1: | 417.1 |
| Heat of atomization / kJ mol-1: | 535.43 |
| First ionization energy / kJ mol-1: | 597.64 |
| Second ionization energy / kJ mol-1: | - |
| Third ionization energy / kJ mol-1: | - |
| in the atmosphere / ppm: | - |
| in the Earth's crust / ppm: | 0.91 |
| in the oceans / ppm: | 0.003 |
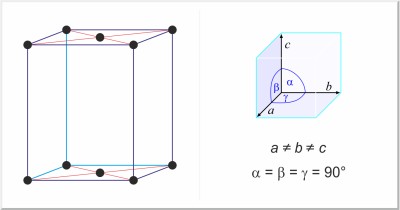
| Crystal structure: | base-centered orthorhombic |
| Unit-cell dimensions / pm: | a=284.785, b=585.801, c=494.553 |
| Space group: | Cmcm |
| Isotope | Relative atomic mass | Mass percent (%) |
|---|---|---|
| 233U | 234.040946(2) | * |
| 234U | 234.040 95(2) | 0.0055(5) |
| 235U | 235.043 93(2) | 0.7200(12) |
| 236U | 236.045 57(2) | * |
| 238U | 238.050 79(2) | 99.2745(60) |
| Balanced half-reaction | Eo / V | |
|---|---|---|
| U4+ + e- → U3+ | - 0.607 | |
| U3+ + 3e- → U(s) | - 1.798 | |
| UO22+ + e- → UO2+ | +0.052 | |
| UO22+ + 3H+ + 2e- → UOH3+ + H2O | +0.299 | |
| UO22+ + 4H+ + 2e- → U4+ + 2H2O | +0.334 | |
| UO22+ + 2e- → UO2(s) | +0.45 | |
| UO2+ + 3H+ + e- → UOH3+ + H2O | +0.546 | |
| UO2+ + 4H+ + e- → U4+ + 2H2O | +0.612 | |
| UOH3+ + H+ + e- → U3+ + H2O | - 0.538 | |
| UO2(s) + 4H+ + 4e- → U(s) + 2H2O | - 1.444 | |
| UO2(s) + 2H2O + 4e- → U(s) + 4OH- | - 2.39 | |
| U3O8(s) + 4H+ + 4e- → 3UO2 + 2H2O | +0.260 | |
| U(OH)3(s) + 3e- → U(s) + 3OH- | - 2.17 |
| 91 Protactinium | ← | 92 Uranium | → | 93 Neptunium |
Citing this page:
Generalic, Eni. "Uranium." EniG. Periodic Table of the Elements. KTF-Split, 18 Jan. 2024. Web. {Date of access}. <https://www.periodni.com/u.html>.
Articles and tables
- Periodic table
- Online calculators
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Preparation of solutions
- Labeling of chemical containers
- Oxidation numbers calculator
- ARS method
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Memory game
- Find the pairs
- Articles and tables
- Chemistry
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Ecology
- Web design
- Chemistry dictionary
- Chemistry
- Downloads
- ≡ Menu
Copyright © 1998-2024 by Eni Generalic. All rights reserved. | Bibliography | Disclaimer
